Electroformed Components
Electroforming Precision Metal Components
ELECTROFORMING is a cost-effective Additive Manufacturing (AM) process that accurately replicates geometric and organic surfaces using copper and/or nickel in an electro-deposition process.
Catalogued OTS (Off the Shelf) Electroformed Components
- Off-the-Shelf Products
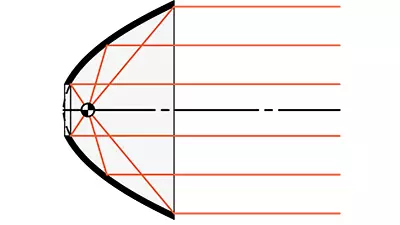
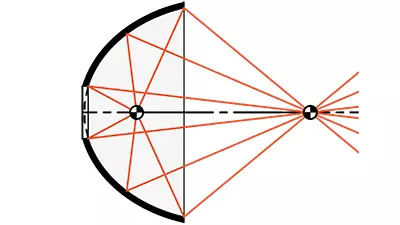
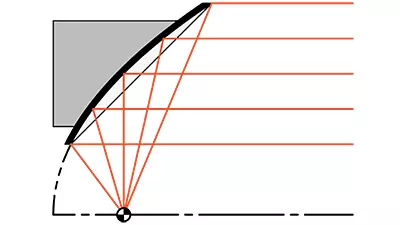
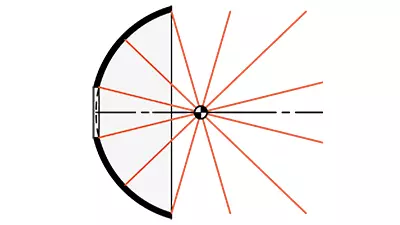
- Nickel (Ni) Metal Reflectors
- Semi-custom and Custom Solutions Available
- Surface Enhancements: Optical Coatings
Customized Electroformed Components
- Nickel (Ni) or Copper (Cu) Metal Substrates
- Simple to Complex Shapes
- Thin to Thick Walled Substrates
- Accurate Reflective Surfaces
- Precision Interior Surfaces
- Structures with “Grown-in” Features
Need a Custom Electroformed Component?
Custom CA and V-DIA Dimensions Available
Related Services
Benefits of Electroformed Components:
- Exact Replicas in Low or High Volume
- Simple to Complex Geometric Shapes
- Organic, Exotic, Lightweight Components
- Thin to Thick Walled Metal Substrates
- Accurate Reflective Surfaces
- Precision Interior Surfaces
- Structures with “Grown-in” Features
ISO 9001:2015 Certified

ITAR Registered

NIST 800:171

SVC Member

NASF Member

SPIE Member